Change Management Software
Reduce the risks of change, improve its implementation, and ensure compliance by using software for Change Control.
With Change Control software, Management of Change (MoC) is quicker, easier, and more reliable.
Automate and control with Change Management Software
Take quality decisions regarding changes in a proactive way. Make adjustments with the least amount of risk and impact possible. Determine, monitor, and document all change risks and their impact on your organisation.
Control the change process effectively regardless of where the issue causing the change arises or how many departments the proposed change affects.
Reduce redundancy and manual labour. Change approvals and notifications regarding any event linked to a change can be automated and managed. Task notifications are automatically issued to the stakeholders who oversee each task. Using industry best practises, tracked and approved modifications may be released and implemented quickly. Easily, with regulatory Change Management Software.
With a good preparation on the design of the forms and workflows, Bizzmine was able to implement both CAPA and MoC application within two days.
Change Management Software for continuous improvement
Any quality item, such as change records, documents, hazards, CAPAs, customer or supplier complaints, audits, and non-conformances, can be linked.
Integrate your training management in your Change Management Software. Any modification to a document or process that requires new training will instantly trigger training, skills, and exams. You can train colleagues on changes as they occur in real time by connecting your complete quality system.
The interaction with the document control module simplifies the integration of required documents and provides full traceability throughout both modules.
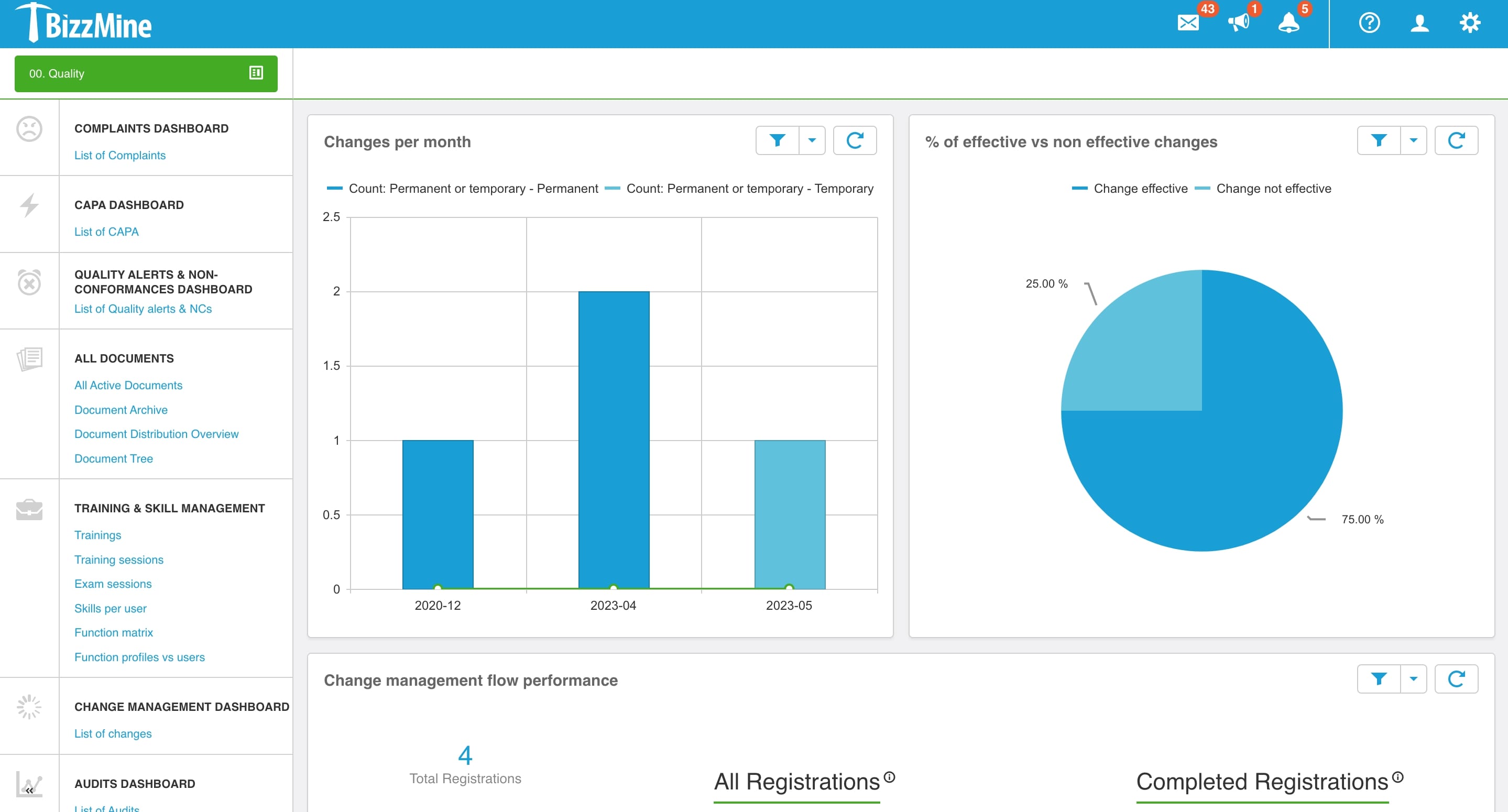
In Bizzmine, we keep all our Quality, Environment, Safety and Sustainability (QHSE) documents. Our processes for managing external and internal audits, Management of Change (MoC), internal non-conformances, customer and supplier complaints, risks and opportunities, meetings, actions, and a number of product developments have been automated with the software.
Reduce the risks of change with Change Management Software
Change Management Software to be compliant and audit ready
Because you can see what was changed, why and when it was changed, and what was impacted by a change, the Change Control process offers complete traceability.
Your quality system is constantly available for inspections and audits, allowing your company to easily meet auditing and compliance needs.
With time-stamped audit trails and electronic signatures, you can stay in compliance with regulatory requirements and standards like ISO and GxP while also meeting FDA's 21 CFR Part 11 and Annex 11 requirements. Easy, with the right regulatory Change Management Software.
Continuously monitor the effectiveness of the change with Change Management Software
Customisable dashboards provide total change visibility for compliance and reporting. Monitor the changes after they are implemented and make notes to analyse the change rollout process and effect to constantly improve your quality approach.
Set up automated procedures and notifications to let IT and business stakeholders understand and communicate more clearly. Handle changes independently with unique and customisable workflows, allowing you to operate the way you want. With automatic notifications in your Change Control Software, you can improve communication.
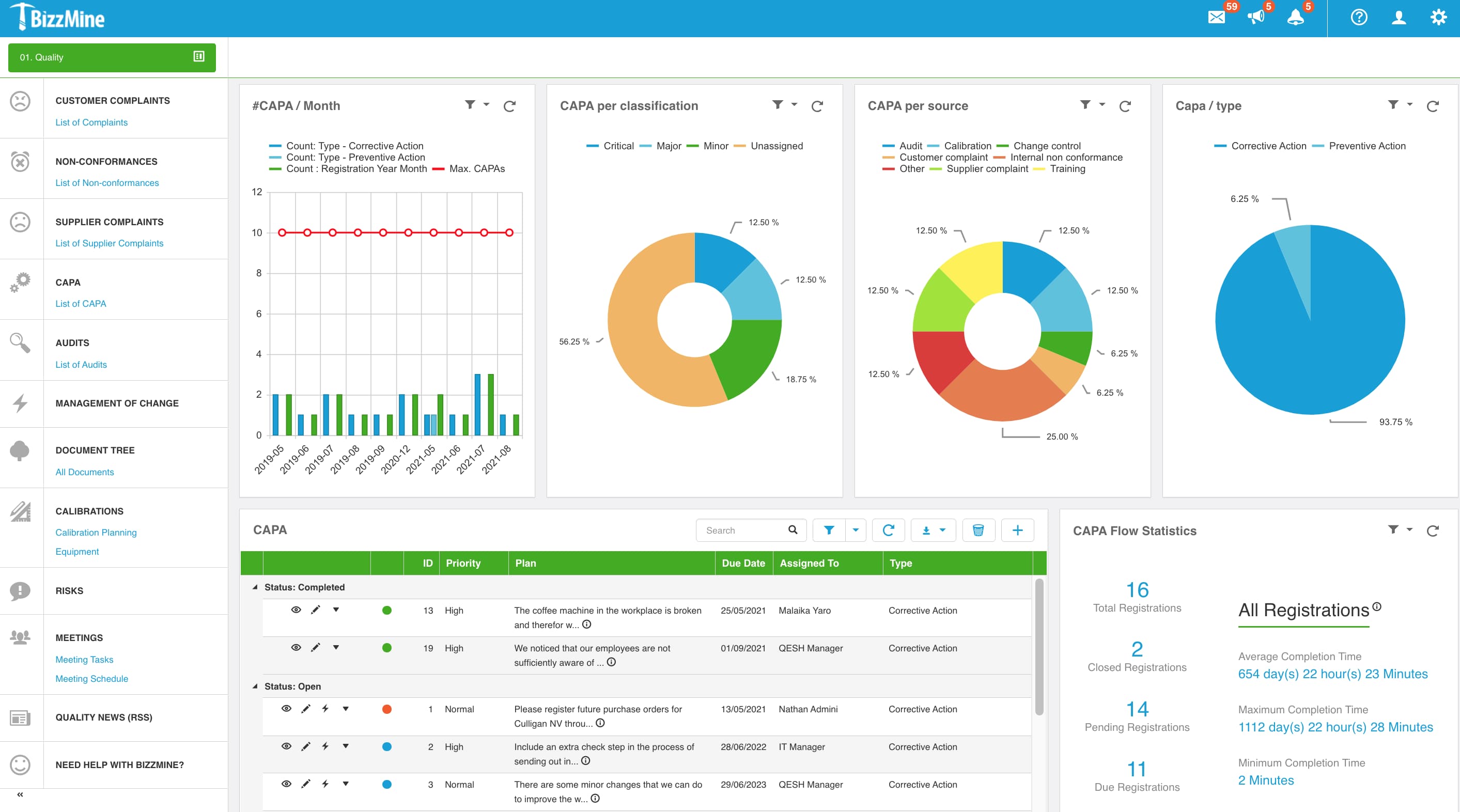
It's very easy to monitor all CAPA and change control processes with the Bizzmine software.
Download your Ebook

Audit Management
Bizzmine assists you in better organising and tracking internal and external audits.

CAPA Management
Bizzmine supports you in continuously improving the efficiency of your QMS.

Training Management
You will be able to better manage and control all training and competency data.

Complaints Management
Keep track of, investigate, and resolve customer and supplier complaints.

Calibration Management
All calibration operations can be planned, organised, and analysed.
Choose your industry and discover more
Medical Devices
See how you can implement your QMS for ISO 13485 and digitise numerous linked quality processes.
Pharma
Discover how to implement your eQMS and digitise numerous linked quality processes, according to cGMP.
Laboratories
Learn how to improve the quality and safety of your laboratory with the implementation of your eQMS.
GDP Logistics
Find out how to organise linked quality processes in accordance with GDP and security requirements.



























