QHSE MANAGEMENT
Transform your Quality and Safety Management with our cutting-edge software.
Streamline processes, enhance compliance, and boost efficiency - all in one scalable platform. Check.Control.Improve.
Pre-validated platform
With Bizzmine QHSE, your quality processes fully comply with ISO 9001, ISO 13485, ISO 17025, ISO 1589, ISO/TS 16949, ISO 22000, 21CFR Part 11, GxP, and GAMP.
The built-in templates ensure all processes and workflows align with regulatory standards.
"Bizzmine brings transparency, compliance and traceability of our Quality registrations."
- Carbogen AMCIS
Discover Bizzmine QHSE now
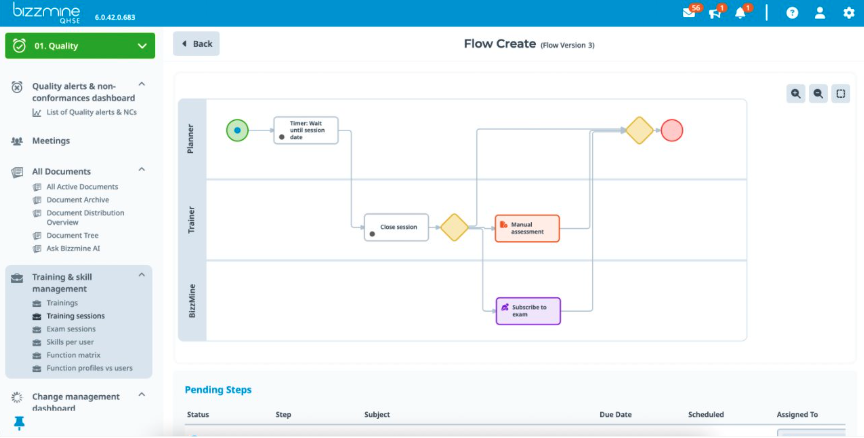
Flexible workflow
The platform allows for extensive customisation - if needed - and enables you to tailor workflows and processes to fit specific industry requirements without complex coding.
Real-time data collection and reporting tools allow you to monitor quality metrics continuously.
Scalable software
Scale from a small business to a large one, spread over multiple sites. The platform grows easily with your business. Expand your digital workflow approach to other departments of your organisation.
Use the software in English, French, Dutch, German, Swedish, Portuguese, Lithuanian, or Italian. More languages can be offered upon request.
SOLUTIONS
Discover comprehensive QHSE solutions to optimise compliance
Document control
CAPA management
Training management
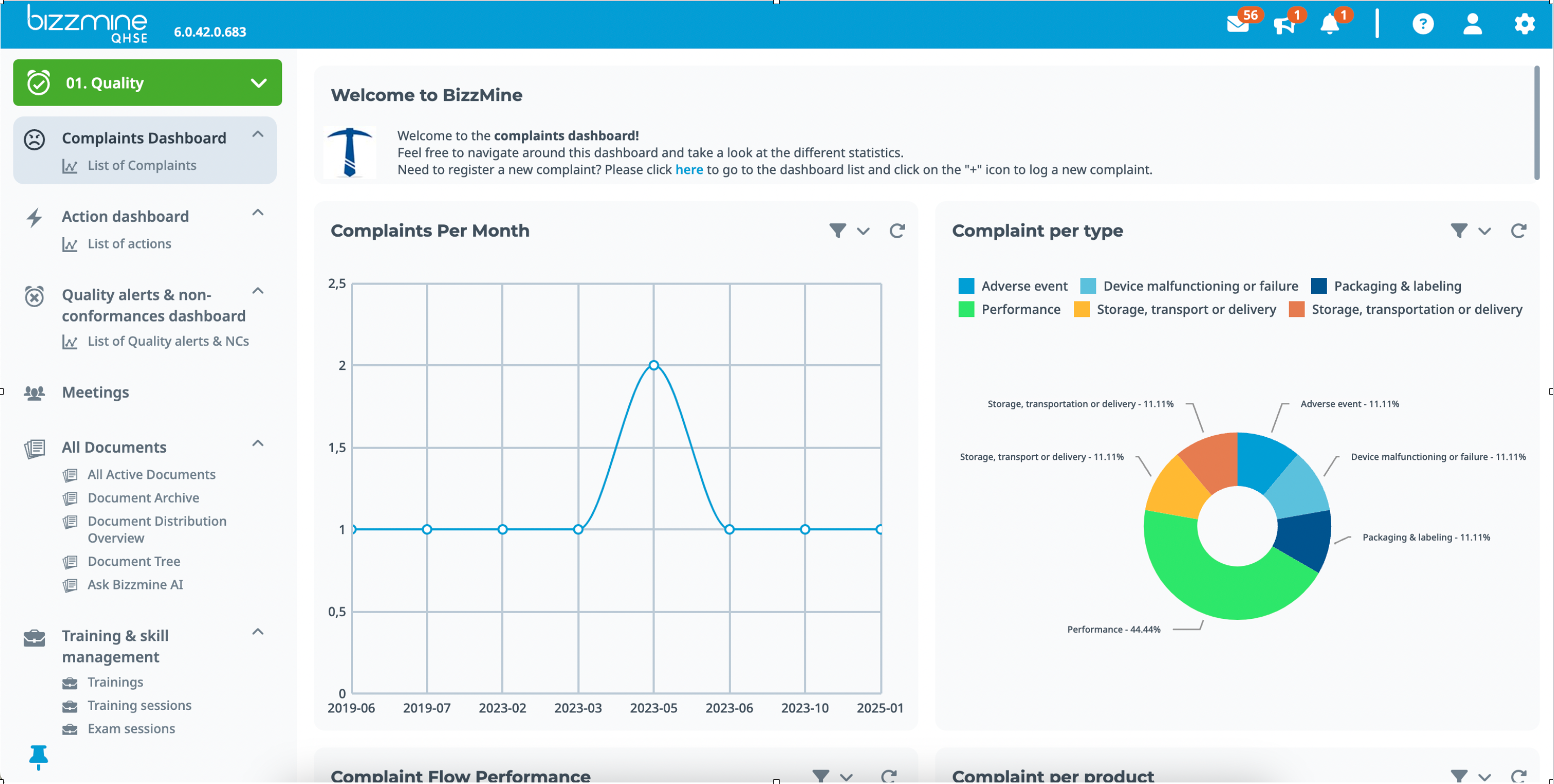
Complaints management
Audit management
Change Control
Calibration management
Supplier Management
Accidents and Incidents
Risk Management
Work Permits
Hazardous Substances
Ebook
12 requirements for a solid digital QHSE Management
A proper system is desirable to manage compliance and streamline operations for continuous improvement. Explore how a software solution can revolutionise QHSE management in any business environment.

%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)

























