CAPA Management System
CAPA management enables you to continuously improve the effectiveness of your quality management system and keep grip on your corrective and preventive actions.
Plan, do, check, act with a CAPA management system
Manage, monitor, and resolve problems inside your company. Non-conformances should be documented throughout the organisation. Capture the problem, analyse it, and implement essential corrective and preventive action to fix it.
Determine, analyse, and track all potential root causes. Conduct thorough risk assessments and establish what steps must be performed.
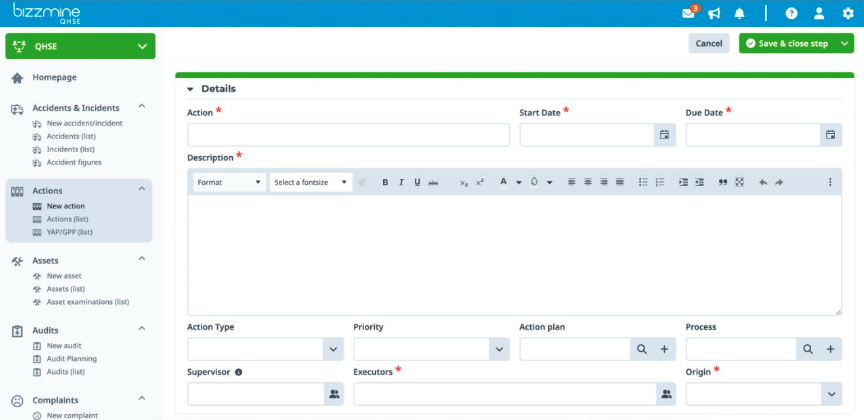
Implement and document the CAPA's outcome. Register all relevant data in the customizable CAPA form, and attach implementation reports or images.
The effectiveness of the CAPA should be measured and validated. If the effectiveness falls short of expectations, the CAPA process's loopback mechanism will allow you to respond and take action.
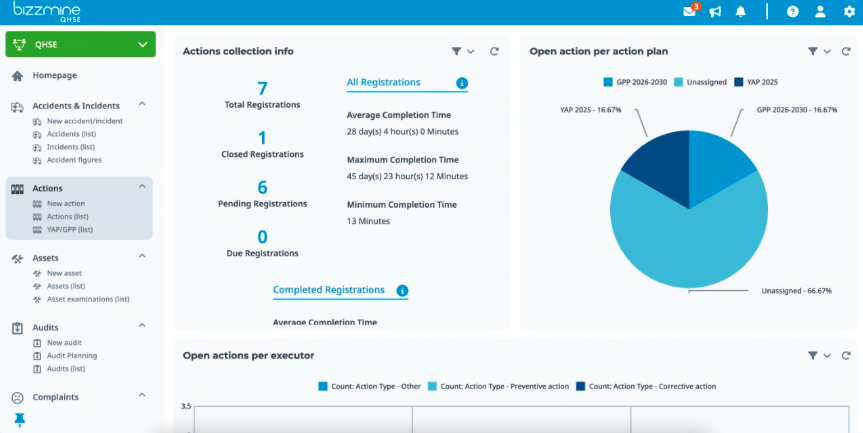
A CAPA Management System to link all CAPA
You can simply generate data on the root causes by working with categories with CAPA management software.
The dashboards are simple and straightforward to use, and they can be customised to display the most important facts.
This enables each group of users to access the overview lists, Pareto graphs, and Trend analyses they require to complete their tasks and fulfil their objectives.
Correct deviations, enable continuous improvement
Benefits of a CAPA management system to monitor your CAPA
Identify, analyse, and track any deviations that may have occurred during an internal or external audit.
To fix and prevent future aberrations, initiate corrective and preventive actions (CAPA).
Task reminder notifications are automatically sent to assigned users and can be issued via e-mail.
"With the Bizzmine workflow module, the CAPA and MoC processes have been made paperless and are compliant with the requirements of 21 CFR Part 11."
- Carbogen AMCIS
Choose your industry to learn more
Medical devices
Pharma
Laboratories
GDP Logistics
Food & Beverages
Unlock your potential with Bizzmine
Change Control
Ensure that changes are properly reviewed, approved, and communicated, preventing unintended consequences or compliance issues.
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)


/Ebook%20cover_Digital%20QMS_EN.png)






















