GDP Logistics Software
How do logistics companies become and stay GDP compliant with a quality management system?
Learn how to become a pioneer in life science logistics.
Ensuring compliance with GDP logistics software is
critical for authorising medicines in the supply chain

Avoid customer claims
Achieve excellence in Pharma Logistics by meeting GDP certification requirements. Use Pharma Compliance Software for managing risks and quality in drug transport. Stay prepared for audits with efficient eQMS, ensuring high customer satisfaction in pharmaceutical logistics.

GDP tailor made
Discover how eQMS, tailored for GDP Pharma logistics, aligns with good distribution practices Guidelines. Our Pharmaceutical GDP logistics software solution offers a customised solution, that ensures strict compliance and effective quality management in pharmaceutical transportation.
OOS analysis
In GDP pharma logistics, OOS (Out-Of-Specification) analysis is vital. Using temperature loggers ensures compliance with storage standards. Good documentation practices are essential for tracking and maintaining product integrity, safeguarding against quality deviations.
"Thank god, that we have never been audited before"
- Logistics companies before they used Bizzmine's GDP Logistics software to be GDP compliant
GDP logistics template database
Our Good Distribution Practices Template Database is designed to get your logistics operations up and running quickly. With scalability at its core, it offers the flexibility of an out-of-the-box solution. Tailored specifically for GDP logistics quality processes, our workflows streamline your operations. Your path to optimised GDP logistics starts with using the right GDP logistics software towards compliance.
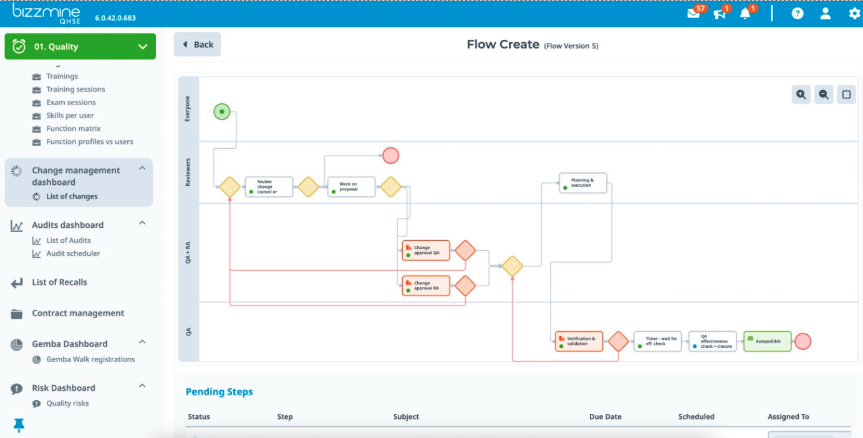
Become GDP certified, and stay it with our GDP logistics software
Our eQMS GDP logistics software is your gateway to achieving and maintaining GDP certification in the pharmaceutical industry. Designed for logistics companies, it ensures ongoing compliance with the highest standards. Attain your good distribution practices certificate efficiently and cost-effectively. Embrace CEIV guidelines and excel in Pharma Logistics. Stay certified, stay ahead.
.jpg)
Compliant at all times with our GDP logistics software
Our GDP Logistics software is built to withstand audits, tailored specifically for logistics. It combines cost-efficiency with the flexibility of a state-of-the-art solution. Security is a top priority, ensuring your operations are protected at all times. Adopt a continuous improvement process with our software, designed for excellence in compliance and efficiency.
.jpg)
Discover our pharma supply chain software for GDP logistics
Meet all good distribution practices certification requirements, handle quality risks, and protect products during their transportation. Streamline your supply chain in life sciences with our efficient GDP compliant software solution.

If you start your day with Bizzmine and you follow the correct procedure, there is no way you can go on working with a wrong or outdated document.
We are strictly bound by GDP regulations and must follow strict rules for documentation. With Bizzmine, we can be sure that we meet these requirements.
Lynn Ackermans - H.Essers


Bizzmine has had a considerable impact for our GDP compliance. The software has made the entire process more streamlined and efficient. Now, all departments are using Bizzmine for full quality management across all functions, and it has transformed the quality culture.
Jenna Cordery - Geodis FF UK LTD


Thanks to Bizzmine, we can do more work with the same number of employees.
With Bizzmine's workflow module, we can establish communication between different departments in a very structured way.
Kevin De Bot - DSV Solutions
Clients about Bizzmine's tailor made GDP logistics software
Pre-validated software solution, tailor made for the logistics industry (GDP).
Whether you have a question about our software, or you'd like to speak to one of our specialists: we got you. You are just one click away from getting in touch with us.
What to expect?
Introduction about the platform and its functions
How our GDP software can help your specific organisation
Personal answer to your detailed questions about GDP compliance
%20(1).webp?width=2000&name=two-happy-businessman-working-laptop%20(3)%20(1).webp)




















